Abstract
Introduction
Mutational profiling in peripheral T-cell lymphoma (PTCL) is increasingly used to aid in diagnosis (Wang. Blood 2015), predicting response (Ghione. Blood Adv 2020), and prognosis (Watatani. Leukemia 2019). However, many analyses lack details of upfront treatment and survival outcomes. The Memorial Sloan Kettering Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) is a custom hybridization capture-based assay encompassing the protein-coding exons of >400 targeted genes (Cheng. J Molec Diag 2015). Using the MSK-IMPACT data generated from a large TCL patient (pt) population (N=396), we sought alterations which may predict resistance to or high rates of relapse after CHOP-based chemotherapy.
Methods
PTCL pts with MSK-IMPACT were detected in the CBioPortal online platform. We included histologies treated with curative intent CHOP-based induction +/- autologous stem cell transplant (ASCT). This included PTCL-not otherwise specified (PTCL-NOS), angioimmunoblastic T-cell lymphoma (AITL), PTCL with a T-follicular helper phenotype (FH-TCL), ALK+ and ALK- anaplastic large cell lymphoma (ALCL), and monomorphic epitheliotropic intestinal T-cell lymphoma (MEITL). Univariate analysis (UVA) for PFS and OS based on the presence of recurring genetic alterations was done using Cox proportional hazard regression analysis. Mutations (mut) assessed included TET2, DNMT3A, RHOA, IDH2, TP53, FAT1, STAT3, STAT5B, JAK1, SETD2, and copy number alterations (CNA) in TP53 and CDKN2A. Comparisons of survival curves were performed by log-rank test. As many pts were sequenced at relapse, to minimize bias, relevant findings were confirmed in the smaller set of pts sequenced at diagnosis and/or before relapse (prospective cohort).
Results
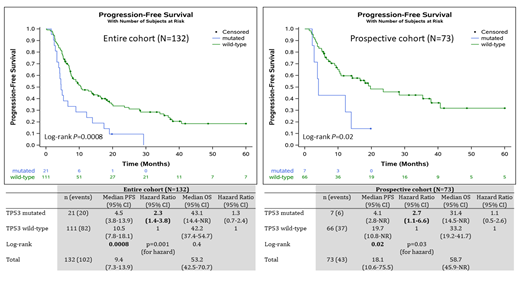
In total, 131 pts met inclusion criteria and had >1 MSK-IMPACT. One pt with a 49-gene panel was also included (N=132). The prospective cohort had 73 (55%) pts. Histologies were PTCL-NOS (N=36, 27%), AITL (N=62, 47%), FH-TCL (N=9, 7%), ALK-ALCL (N=15, 11%), ALK+ALCL (N=6, 5%), and MEITL (N=4, 3%). Regimens were CHOP + etoposide (N=59, 45%), CHOP (N=40, 30%), brentuximab + CHP (N=15, 11%), other CHOP-based (N=18, 14%). The most frequent mut were TET2 (N=69, 52%), RHOA (N=40, 30%), DNMT3A (N=25, 19%), TP53 (N=21, 16%), and IDH2 (N=15, 11%), and for CNA were losses of TP53 (N=9, 7%) and CDKN2A (N=9, 7%). TET2 mut were most frequent in AITL (N=51, 82%), FH-TCL (N=6, 67%), and PTCL-NOS (N=10, 28%). RHOA mut were found in 2 cases of PTCL-NOS (6%) with the rest in AITL (N=33, 53%) and FH-TCL (N=5, 56%). DNMT3A mut were most frequent in AITL (N=19, 31%) and FH-TCL (N=4, 44%). IDH2 mut were exclusive to AITL (N=14, 23%) and FH-TCL (N=1, 11%). TP53 mut were found in all histologies: ALK-ALCL (N=5, 33%), PTCL-NOS (N=10, 28%), FH-TCL (N=2, 22%), AITL (N=2, 3%), and in one case each of ALK+ALCL (17%) and MEITL (25%). The 24-month PFS was 28% for the entire cohort, and 42% for the prospective cohort. On UVA for genetic alterations, only TP53 mut (P=0.0011) and TP53 deletions (P=0.009) associated with inferior PFS. On MVA, only TP53 mut remained significant (HR 2.0 [95% CI 1.1-3.5] P=0.02). No alteration associated with inferior OS. For the entire cohort, median PFS was 4.5 mos for TP53 mut (N=21) vs. 10.5 mos for TP53 wild-type (WT) (N=111) (log-rank P=0.0008). This was similar in the prospective cohort with a median PFS of 4.1 vs. 19.7 mos (log-rank P=0.02) (Figure 1). Compared to TP53 WT, TP53 mut were more likely to have PTCL-NOS (P=0.03), concurrent deletions of TP53 (P=0.0005), and a higher median number of alterations (P=0.01). There were no differences in age, stage, marrow disease, or IPI/PIT scores between TP53 mut and TP53 WT pts. There was a trend towards fewer CR (P=0.054) in TP53 mut. There were no differences in ITT with ASCT between the groups (P=0.5). This was similar in the prospective cohort (P=0.4). Six total (29%) TP53 mut received ASCT, and PFS was similar to ASCT in TP53 WT (median 18.2 vs. 19.7 mos, P=0.5), but 5/6 (83%) ultimately relapsed.
Conclusions
TP53 mut correlated with lower rates of CR, higher rates of relapse, and shorter PFS in this dataset of PTCL treated with CHOP-based chemotherapy. OS was not different compared to TP53 WT tumors. The confounding impact of histology and other prognostic factors as well as the lack of uniform prospective mutational profiling in this retrospective series precludes definitive conclusions and requires prospective confirmation.
Sauter: Juno Therapeutics: Consultancy, Research Funding; Sanofi-Genzyme: Consultancy, Research Funding; Spectrum Pharmaceuticals: Consultancy; Novartis: Consultancy; Genmab: Consultancy; Precision Biosciences: Consultancy; Kite/Gilead: Consultancy; Celgene: Consultancy, Research Funding; Gamida Cell: Consultancy; GSK: Consultancy; Bristol-Myers Squibb: Research Funding. Khan: Seattle Genetics: Research Funding. Moskowitz: ADC Therapeutics: Research Funding; Beigene: Research Funding; Seattle Genetics: Consultancy, Research Funding; Miragen: Research Funding; Bristol-Myers Squibb: Research Funding; Merck: Consultancy, Research Funding; Janpix Ltd.: Consultancy; Imbrium Therapeutics L.P./Purdue: Consultancy; Takeda: Consultancy; Incyte: Research Funding. Dogan: Seattle Genetics: Consultancy; Roche: Consultancy, Research Funding; Peer View: Honoraria; EUSA Pharma: Consultancy; Takeda: Consultancy, Research Funding; Physicians' Education Resource: Honoraria. Horwitz: ADC Therapeutics: Consultancy, Research Funding; Acrotech Biopharma: Consultancy; Affimed: Research Funding; Aileron: Research Funding; Celgene: Research Funding; C4 Therapeutics: Consultancy; Daiichi Sankyo: Research Funding; Forty Seven, Inc.: Research Funding; Kyowa Hakko Kirin: Consultancy, Research Funding; Janssen: Consultancy; Kura Oncology: Consultancy; Millennium /Takeda: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Trillium Therapeutics: Consultancy, Research Funding; SecuraBio: Consultancy, Research Funding; Myeloid Therapeutics: Consultancy; ONO Pharmaceuticals: Consultancy; Shoreline Biosciences, Inc.: Consultancy; Tubulis: Consultancy; Vividion Therapeutics: Consultancy; Verastem: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal